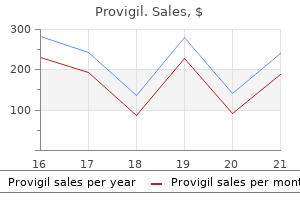
Provigil dosages: 200 mg, 100 mg
Provigil packs: 30 pills, 60 pills, 90 pills, 120 pills, 180 pills, 270 pills, 360 pills

Discount provigil 100 mg visa
The coaching procedure should simulate applicable worst-case circumstances related to the product residues which may be sampled on the web site insomnia sign of pregnancy 100 mg provigil generic with mastercard. The coaching should embrace applicable quantitative acceptance criteria to show the competence of the trainee sleep aid zeppelin purchase provigil 100 mg otc. Periodic recertification of personnel is recommended to reinforce and maintain the manual dexterity and abilities required for swab sampling. If an auxiliary implement similar to an extension pole is used in sampling, the training Cleaning Validation Lifecycle Stage 2-Process Qualification Application of Process Validation Stage 2 Process Qualification ideas to cleaning validation includes work performed to demonstrate and ensure a profitable cleaning course of. Validation efficiency contains the activities and documentation to demonstrate the successful growth of a new process or the profitable implementation of change to a validated course of. These activities are primarily based on the Stage 1 course of understanding work described previously. After cleaning, equipment is inspected and tested to prove that acceptable degree of soil has been attained. The work of Stage 2 ought to be commensurate with the identified or expected threat related to cleaning. Many organizations erroneously consider validation efficiency to be the total idea of validation. The efficiency of multiple full-scale conformance lots confirms the acceptability of manufacturing processes as previously designed and developed. All cleaning tools must be qualified when utilized in Stage 2 course of qualification. All analytical strategies, together with instrumentation, have to be qualified when utilized in Stage 2 course of qualification. A plan for sustaining the cleaning validated state, Stage 3 of cleaning course of validation, should be mentioned within the final validation report. Parenteral Medications that validation checks will meet their predetermined acceptance standards. The approved cleaning validation protocol should be executed with an expectation of complete success. Techniques, sampling, testing, and associated activities should have been tried before being utilized in validation. Validation efficiency must affirm the acceptability of the cleaning process as previously developed. Completion of Stage 1 Technical Development Work All work supportive of the final cleaning process should be completed prior to initiation of Stage 2 Process Qualification. Completion of the aforementioned actions contains completed documentation describing all of the above. The work described in these initiatives must instantly assist efficiency of the cleaning process, tools and utilities used in the course of, and sampling and testing required to validate the process. The aforementioned documentation should be referenced in cleaning validation documentation and be readily available for audit as needed. Considerations Prior to Cleaning Process Performance Initiation of Stage 2 Process Qualification is a crucial milestone in the cleaning validation. Initiation of Stage 2 Process Qualification implies that the cleaning course of has been totally developed. Stage 2 cleansing course of performance should affirm the cleaning process development work. There must be good confidence Master Cleaning Procedure Record the final word result of Stage 1 cleansing course of growth is the Master Cleaning Procedure Record. This document is equivalent to the master manufacturing batch report containing all manufacturing process steps for a pharmaceutical product. A copy of the Master Cleaning Procedure Record is issued each time the cleaning process is utilized. The particular procedural steps and course of parameters in the Master Cleaning Procedure Record ought to be addressed and justified within the cleaning course of development report. The Master Cleaning Procedure Record should embrace detailed process steps in the manner of a Cleaning Validation-Lifecycle Approach manufacturing batch report. Details should embody quantitative preparation of cleaning agent liquids, equipment disassembly, quantitative process steps, and different relevant steps in the cleaning process. Performance of all related steps ought to be signed and dated by personnel performing these steps demonstrating accountability for exact efficiency of the desired process. The Master Cleaning Procedure Record must affirm that cleaning is a specified process that must be fastidiously accomplished and never an activity during which personnel "do no matter it takes to get the job carried out. Sampling, testing, and acceptance criteria justification and rationale are mentioned in these paperwork. The validation initiation/plan and validation protocol are permitted by the positioning validation approval committee. This activity represents the culmination of all course of understanding work, including R&D, scale-up, technology transfer, and commercial-scale improvement. These paperwork should be a logical extension and consistent with Stage 1 development documents demonstrating the scientific and technical basis for the cleansing process. This activity represents the end result of all process understanding work, together with R&D, scale-up, expertise switch, and commercial-scale improvement of the cleansing process. It consists of actual execution of the cleansing process to be validated, sampling, and related testing. The following are discussed: � Preparation for cleansing validation � Conformance lot cleaning-Execution of the cleaning process report � Conformance heaps sampling and testing. Cleaning Procedure Records for Conformance Lots the cleaning procedure data for conformance lots are copied from the Master Cleaning Procedure Record. A copy of the Cleaning Procedure Record should be included in the cleansing validation plan or protocol to specifically establish the method being validated. Preparation for Cleaning Validation Preparation for cleansing process validation includes actions related to cleansing course of performance. Personnel who shall be cleansing equipment or operating automated gear must be succesful of appropriately executing the cleansing process. Also included are actions related to cleansing equipment performance, including readiness of procedures, calibration, and preventive upkeep. These include cleaning after a number of heaps in a manufacturing campaign, time between completion of producing and begin of cleansing ("soiled maintain time"), and different variables doubtlessly affecting cleansing performance. All affected teams must concentrate on the approaching conformance tons, together with sampling personnel, laboratory testing personnel, and different associated capabilities. Validation Initiation/Plan and Validation Protocol for Specific Cleaning Process Validation these are commonplace validation paperwork utilized by the site to doc validation. Typical documents embrace a validation initiation doc that simply describes the validation work to be performed. Coincident with validation initiation is a descriptive plan describing the general necessities to accomplish the specific cleaning validation. This doc ought to provide a great understanding of the target of the validation and all needed requirements (technical, procedures, documentation, and so on.
100 mg provigil buy with amex
In the quenching process sleep aid prescription discount provigil 200 mg free shipping, the polymer/solvent combine is applied onto a drum or belt insomnia pictures buy discount provigil 200 mg, which immerses right into a solvent or extraction bath. Melt extruded films are stretched under outlined course of situations to create a thin membrane. The thinnest (10�20 �m) membrane movies are created by track-etched manufacturing course of. Types of Filters Membrane Filters Membrane filters generally have a defined pore structure and porosity band. The narrower the porosity band, the extra defined the retention fee of such membrane is. The filtration obtained by means of such membrane filters is often referred to as microfiltration. Microporous membrane filters have a much more defined porosity than is out there inside prefilter matrixes. Membranes are produced by either an evaporation, quenching, stretching, or track-etched process. The pore construction of track-etched membranes may be very defined, however due to the avoidance of particle observe overlaps, the porosity is low. Membrane filters could be fashioned in quite so much of buildings for specific application functions. For instance, the formation of uneven membrane constructions, by which the pore size on the upstream facet is larger than the downstream aspect of the membrane, can improve the dust load capability of such filter. Membrane filters are the most typical filtration devices used in aseptic processing to remove organisms from liquids or gases. Due to the outlined structure, these filters are highly dependable with respect to the retention requirements and moreover could be integrity examined. For instance, polycarbonate track-etched filters are used to scale back the size of liposomes and emulsions as a outcome of a well-defined pore structure. Parenteral Medications by the thickness of the person fiber or the compactness of the matrix. Therefore, prefilter types have a large selection and could be chosen for lots of kinds of software. These therapies prevented the release of particulate matter and have been utilized to stabilize the ultimate filter fleece. Additionally, these applied sciences allowed fleece development of various fiber diameters within a filter matrix to be produced. This improved the entire throughput efficiency of those filters as a outcome of fractionate retention of a large spectrum of particle sizes. A additional advance in depth filter technology occurred with the appearance of the primary melt-blown kind of cartridge that included numerous fiber diameters because the filter was manufactured to achieve a graded pore design by means other than various the fiber packing density. In this process, the polymer is extruded via a multi-hole die, and the polymer stream is stretched and attenuated by a high-velocity heated airstream. The mean fiber diameter is changed as the filter is being made by adjusting the air velocity or one of the different variables that contribute to the formation of the fiber sizes, for example, temperature or polymer pumping rate. This know-how is turning into more advanced, with some producers naming the fibrous fleece constructions as nano-fiber fleeces. The concept of using a graded or altering pore size to enhance filtration efficiency is a fascinating one. This method involves incorporating a collection of prefilters right into a single stage to maximize using the complete filter and lengthen filter life (dirtholding capacity). The issue of fractionate retention is particularly important for applications with a wide particulate spectrum, for example, water pretreatment. Prefilters also can contain membranes, porous, or fibrous, commonly from cellulose, mixesters, or borosilicate. These prefilter types are utilized to remove a very fantastic band of particulate or contaminants from the fluid to particularly shield sterilizinggrade membrane filters. Highly adsorptive cellulosic or kieselgur containing depth filter pads are welded collectively in a plate format. These plate formats commonly have a diameter of 12 or 16 and contain stacks of 4�16 to create a depth filter unit. The adsorptive depth filter materials is right to separate colloidal substances and lipids; consequently, these filters have been very often utilized in plasma and serum purposes. Nowadays, lenticular filters are most frequently used in cell harvest functions after the fermentation process to separate cell debris and different contaminants. When lenticular filter combinations are tested, the checks not only contain the entire throughput of the filter element as is often the case with pleated prefilter cartridges, however an important issue is the turbidity measurement of the filtrate. The turbidity measurement will create an indication of the protecting properties of the lenticular filter retention score used and how much of the contaminants are separated by the actual filter score. Small-scale exams are carried out to decide which filter combination is good and meets the process necessities. The report also addresses the problem of the release of beta glucans from a variety of the depth filter supplies, which might create a false failure in the endotoxin take a look at ranges. The filters require thorough flushing to keep away from any undesired leaching into the product stream. Prefilters Prefilters are mostly depth filter varieties and are generally constructed of nonwoven or melt-blown fiber materials such as polypropylene, polyamide, cellulosic, glass fiber, steel fibers, and sintered chrome steel (12). Normally, prefilter materials are constructed into mats by the random deposition of either particular person or steady fibers whose fixation is completed by urgent, heating, gluing, entanglements, or other forms. The pores of such filter constructions are quite random interstices among the fibers. The casting answer consists of polymer dissolved in a mix of solvent and high-boiling non-solvent. Pore formation happens as follows: as solvents progressively evaporate from the casting resolution, the non-solvent will increase in content material to the point the place part separation takes place. Non-solvent droplets separate within the polymer/solvent section, and polymer comes out of resolution to concentrate on the droplet interfaces. The swollen polymer shells surrounding the non-solvent droplets thicken as continuing solvent loss causes more polymer deposition. The eventual disappearance of the polymer/solvent phase brings the polymer-surrounded droplets into mutual contact. They consolidate into clusters and deform into polyhedral cells crammed with nonsolvent beneath the impetus of the realm minimizing forces. Finally, the perimeters of the cells accumulate polymer on the expense of the cell walls. Thinning of the partitions of the polyhedra results in their rupture and interconnection. The reticulation of the discrete cells of the polymeric matrix permits the removing of the non-solvent, as by washing. Not the polyhedral cells, however their interconnecting openings, thus shaped, comprise the metering pores of the membrane (15). Nanofilters Most generally, nanofilters are designed to separate viruses, using dimension exclusion because the predominate mechanism of removing. Since nanofilters are extraordinarily tight filters, the water bubble level is commonly greater than the utmost allowable operating strain.
Diseases
- Orofaciodigital syndrome type1
- Myalgia eosinophilia associated with tryptophan
- Turner-like syndrome
- Adams Oliver syndrome
- Hyperreflexia
- Polydactyly postaxial with median cleft of upper lip
- Pica
Provigil 200 mg buy cheap on line
It is essential to understand the symptoms that the patient considers to represent constipation sleep aid vape juice buy 100 mg provigil with visa. Dietary insomnia vegas provigil 200 mg visa, hydration, and physical exercise habits should also be decided to identify potential contributing factors. Family historical past should be assessed for the presence of inflammatory bowel illness and colon most cancers. A full report of prescription drugs, over-the-counter products, and dietary supplements is necessary to identify drugrelated causes. The diagnosis of constipation relies on scientific history, physical examination, minimal laboratory tests, colonoscopy or different procedures, and specific tests to consider underlying pathophysiology. Endoscopy is required in sufferers with weight reduction higher than 10 kilos in three months, rectal bleeding, or anemia to exclude most cancers or strictures, particularly in sufferers older than 50 years. Anorectal examination, manometry, radiography, colonoscopy, and other procedures could additionally be helpful in certain circumstances. L O 2 In most cases, the bodily examination is normal, and no underlying explanation for constipation is recognized. Nonpharmacologic Therapy In many circumstances nonpharmacologic remedy together with lifestyle and dietary modifications should be employed previous to recommending pharmacotherapy. Patients should be encouraged to schedule routine rest room time after the morning or night meal. High-fiber foods embody beans, whole grains, bran cereals, fresh fruits, and greens corresponding to asparagus, brussels sprouts, cabbage, and carrots. Some sources of soluble fiber embrace lentils, apples, nuts, flaxseed, and psyllium. Some sources of insoluble fiber embrace entire wheat, corn bran, couscous, dark leafy green vegetables, and root vegetable skins. Adequate fluid intake is essential, particularly in sufferers with evidence of quantity depletion. The thirst mechanism modifications with age; keeping a daily consumption diary might assist patients who have to be reminded to drink fluids. Patients are guided to demonstrate the flexibility to coordinate belly and pelvic floor motion throughout evacuation. Colectomy and ileorectal anastomosis may be thought-about in select patients with slow-transit constipation. They act by swelling in intestinal fluid, forming a gel that aids in fecal elimination and selling peristalsis. They may trigger flatulence (less generally with methylcellulose), bloating, distention, and belly cramping. Fiber dietary supplements may end in much less enchancment in patients with delayed colon transit constipation and/or obstruction. Bulk-forming or fiber laxatives must be taken with sufficient water (240 mL/dose) to avoid becoming lodged in the esophagus and producing obstruction or worsening constipation. Hypersensitivity reactions might happen and barely may be manifested as an anaphylactic reaction. These products trigger water to enter the lumen of the colon and may Magnesium can accumulate in renal dysfunction. [newline]Lactulose acidifies colonic contents, increases water content material of the gut, and softens the stool. Sorbitol can cause intestinal irritation and will have an result on blood glucose levels in diabetic sufferers. In patients with kidney disease this over-the-counter product should be used solely on the recommendation and supervision of a doctor. The oily film masking the stool also retains the stool from shedding its water to intestinal reabsorption processes. Oral mineral oil (liquid petrolatum) is a nonprescription heavy oil that ought to be used with caution, if in any respect, as a outcome of it might be aspirated into the lungs and trigger lipoid pneumonia. Intestinal Secretagogues Lubiprostone (Amitiza) this oral agent is derived from prostaglandin E1 and acts domestically on intestinal chloride channels to increase intestinal fluid secretion, leading to increased intestinal motility and passage of stool. Adverse effects of lubiprostone include dyspnea, nausea, diarrhea, abdominal distention and pain, flatulence, vomiting, and free stools. Adverse results of linaclotide include diarrhea, headache, fatigue, dehydration, belly pain, flatulence, and abdominal distention. Loose stools and larger stool frequency might occur after administration with a high-fat breakfast. It is contraindicated in kids underneath the age of 6 years as a end result of increased fluid secretion might lead to critical dehydration. Enteric-coated bisacodyl tablets should be swallowed entire to avoid gastric irritation and vomiting. Ingestion should be averted within 1 to 2 hours of antacids, H2-receptor antagonists, proton pump inhibitors, and milk. The onset of motion of stool softeners is longer than with most stimulants and will take as a lot as seventy two hours. Saline Agents Salts of sodium, magnesium, and phosphate pull water into the lumen of the intestines resulting in increased enteral stress. Principal considerations with sodium phosphate derivatives include dehydration, hypernatremia, hyperphosphatemia, acidosis, hypocalcemia, and worsening renal function. Elderly people and patients with heart failure and renal dysfunction should be suggested to keep away from saline agents. Nonprescription oral sodium phosphate options are not out there because of the chance of acute phosphate nephropathy, a form of acute kidney damage. For adults with swallowing difficulties, the tablets may be crushed and administered orally both in applesauce or with water or given with water through a nasogastric or gastric feeding tube. The major antagonistic impact related to plecanatide is diarrhea, which may lead to discontinuation in some sufferers. Less widespread adverse results include sinusitis, higher respiratory tract infection, belly distention, flatulence, stomach tenderness, and elevated hepatic enzymes. The really helpful dose is 25 mg orally once every day on an empty stomach no much less than 1 hour prior to the first meal of the day or 2 hours after the meal. Naldemedine (Symproic) is a by-product of naltrexone that contains an added aspect chain that will increase molecular weight and reduces its capacity to cross the blood�brain barrier. Naldemedine is also a substrate of the P-glycoprotein (P-gp) efflux transporter; sufferers taking P-gp inhibitors (eg, amiodarone, captopril, cyclosporine, verapamil) ought to be monitored for adverse results as a result of elevated naldemedine concentrations. Patients receiving opioids for less than 4 weeks may be less aware of these brokers. Symptoms of opioid withdrawal may occur (sweating, chills, diarrhea, belly pain, nervousness, yawning) in sufferers physically dependent on opioids. Methylnaltrexone Bromide (Relistor) is a quaternary amine derivative of naltrexone. Treatment Recommendations the standard efficient, safe, and inexpensive modalities (fluid consumption; dietary and supplemental fiber; stool softeners; and saline, stimulant, or osmotic laxatives) ought to be attempted before agents such as secretagogues are prescribed. Pregnant girls ought to be suggested to eat regular meals which are balanced among fruits, vegetables, and complete grains and maintain adequate water consumption to avoid constipation. Magnesiumbased antacids have minimal absorption and are considered low danger in pregnant ladies. Children younger than 6 years old should be evaluated by a healthcare provider before being given a laxative as a outcome of they might not have the flexibility to describe their signs well.
200 mg provigil buy free shipping
If a affected person is vulnerable to movement sickness sleep aid 2012 provigil 200 mg on line, preventive measures embody minimizing publicity to motion insomnia netflix 100 mg provigil order with mastercard, proscribing visible exercise, ensuring enough air flow, lowering the magnitude of movement, and taking part in distracting activities. Oral medications must be taken prior to motion exposure to enable time for sufficient absorption. Once nausea and vomiting because of motion sickness occur, oral medicine absorption may be unreliable, making the therapies ineffective. Pyridoxine is properly tolerated, however doxylamine and different antihistamines may trigger drowsiness. Although the safety of anticholinergics and metoclopramide have been established, data demonstrating efficacy are missing. Because methylprednisolone is associated with oral clefts in the fetus when used during the first trimester, corticosteroids ought to Patient Encounter three A 70-year-old girl who presents to your follow is planning a 10-day Mediterranean cruise. She has experienced nausea and vomiting throughout boat rides up to now and states that a good friend recommended she buy "a patch" at her local pharmacy to stop nausea and vomiting. What suggestions for nonpharmacologic interventions would you give this affected person to assist forestall movement sickness What are the pharmacologic options for this affected person to stop or treat nausea and vomiting Assess the Information: � Assess the patient to determine whether or not the nausea and vomiting is easy or complex and whether patientdirected therapy is acceptable. Develop a Care Plan: � Eliminate the underlying explanation for nausea and vomiting if possible. Follow-up: Monitor and Evaluate: � To assess efficacy, ask the patient whether nausea or vomiting is resolving with remedy. Assess whether or not remedy failure is due to inappropriate medicine use or the necessity for extra or completely different treatments and proceed accordingly. Antiemetics: American Society of Clinical Oncology scientific follow guideline replace. Chemotherapy-induced nausea and vomiting: an summary and comparability of three consensus tips. Haloperidol versus ondansetron for prophylaxis of postoperative nausea and vomiting. Pathophysiology and pharmacotherapy of gastroparesis: current and future views. Pharmacological features of anticancer drug-induced emesis with emphasis on serotonin launch and vagal nerve activity. Chemotherapy-induced nausea and vomiting: antiemetic trials that impacted clinical apply. Palonosetron in the administration of chemotherapy-induced nausea and vomiting in patients receiving multiple-day chemotherapy. Management of chemotherapy-induced nausea and vomiting: focus on newer brokers and new uses for older brokers. Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: a scientific evaluation. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomized, active-controlled, double-blind, part 3 trials. Delayed chemotherapy-induced nausea and vomiting: pathogenesis, incidence, and current administration. Efficacy of acupressure and acustimulation bands for the prevention of movement illness. Stimulation of the wrist acupuncture point P6 for stopping postoperative nausea and vomiting. The impact of transdermal scopolamine for the prevention of postoperative nausea and vomiting. Prochlorperazine versus promethazine for uncomplicated nausea and vomiting in the emergency division: a randomized, double-blind scientific trial. The effectiveness of rescue antiemetics after failure of prophylaxis with ondansetron or droperidol: a preliminary report. Recommend life-style modifications and pharmacotherapy for remedy of constipation. Thorough patient evaluation is essential to precisely establish the underlying cause and implement protected and effective remedy. As a result, many diagnostic exams (eg, x-rays, endoscopic examinations) are sometimes negative. Dyssynergic defecatory or rectal evacuation disorders contain prolonged rectal storage of fecal residue or issues of evacuation with regular or delayed colonic transit leading to incomplete expulsion of feces from the rectum. Laboratory Tests (to Identify Secondary Causes) � Thyroid-stimulating hormone (hypothyroidism) � Serum calcium (may be elevated or decreased) � Glucose (diabetes mellitus) � Serum electrolytes (dehydration, quantity depletion) � Urinalysis (dehydration) � Complete blood rely (anemia) Other Tests � Screening colonoscopy and different specialised tests may be carried out based mostly on presenting signs and signs. Activation of these receptors reduces acetylcholine release, which contributes to physiologic adjustments similar to decreased propulsive exercise; elevated nonpropulsive contractions; decreased pancreatic, biliary and gastric secretions; and elevated anal tone. The medical staff decided that he suffered no serious damage and had no cognitive impairment. All laboratory exams have been inside the reference ranges, and the patient was discharged to home. What nonpharmacologic and pharmacologic therapies could be applicable for his condition Patient Care Process for Constipation Collect Information: � Ask about signs to decide if patient-directed therapy is appropriate or if the affected person must be evaluated by a doctor. Determine sort, frequency, and length of signs; presence or absence of abdominal ache; and exclude the presence of alarm symptoms. Assess the Information: � Obtain an intensive historical past of prescription medicine, nonprescription product, and dietary complement use. Determine what remedies have been helpful prior to now, whether or not the affected person is taking any medications that may contribute to constipation, traditional food plan, and fluid intake. Assess for the presence of adverse drug reactions, drug allergic reactions, and drug interactions. Follow-up: Monitor and Evaluate: � Develop a plan to assess the effectiveness of laxative use. Because many older persons experience constipation, laxative use is usually viewed as a traditional a part of every day life. However, oral ingestion of mineral oil can present a specific hazard to bedridden people as a end result of inhalation of oil droplets can lead to pneumonia. Regular use of any laxative that impacts fluid and electrolytes may result in significant antagonistic effects. Patients with the following circumstances ought to use laxatives only under the supervision of a well being care supplier: (a) colostomy; (b) diabetes mellitus (some laxatives comprise sugars corresponding to dextrose, galactose, and/or sucrose); (c) heart illness (some merchandise contain sodium); (d) kidney disease; and (e) swallowing difficulty (bulk-formers could produce esophageal obstruction). Saline laxatives containing magnesium, potassium, or phosphates ought to be used cautiously in individuals with decreased kidney perform. Monitor acceptable serum electrolyte concentrations in patients with unstable renal perform evidenced by changing serum creatinine or creatinine clearance. Patients ought to have an increase in stool frequency to three or extra well-formed stools per week.
Provigil 200 mg
If merchandise containing sulfamethoxazole were cleaned utilizing an acid cleaning agent sleep aid jitters generic provigil 100 mg line, solubility in acid pH ought to be considered insomniac games provigil 100 mg buy overnight delivery. If products containing sulfamethoxazole had been cleaned using water, solubility at neutral pH ought to be thought of. Knowledge of the compound pKa (or multiple pKa), the pH at which 50% ionization happens, is key information. The number of a worst-case compound for cleaning validation is a crucial element of any cleansing program that helps the cleaning of multiproduct equipment. These assessments must be rigorously made contemplating all relevant technical data, together with the physical and chemical properties of the residues. Incorrectly identifying worst-case soils for cleansing validation undermines the complete cleaning program and exposes a corporation to significant regulatory threat. If a worst-case compound is incorrectly chosen, the cleansing validation of all residues in the matrix may be compromised. This data along with compound structure, pKa, and different pharmaceutics information provides useful data for worst-case residue choice. Much of this data is at all times developed throughout new drug and new product growth. This data is valuable for screening drug candidates, prediction of biopharmaceutical properties, formulation improvement, dissolution testing, and different functions. Cleaning professionals ought to work together with pharmaceutics scientists to be certain that their technical wants are met when this fundamental pharmaceutics improvement work is done. A slight improve in solubility occurred in alkaline surfactant liquid (typical cleansing agent). Solubility in the cleaning agent liquid must be the first consideration in determining the worst-case compound for cleansing validation. However, cleaning of the entire product matrix, together with slow-dissolving polymers or different problem-inactive components, may profoundly affect the selection of cleaning agent. Regulatory concerns: A matrix approach to cleansing is acceptable when the matrix is nicely outlined and considers many parameters in defining the matrix. However, other parameters that ought to receive consideration embody the following: � � � � Pharmacological aspects of the product Known toxicity Potency based mostly upon the dose of the energetic substance Quantity or share of active substance within the formulation � Release mechanism of the product to be cleaned. Parenteral Medications highly prudent to verify the matrix theory by performing cleansing verification studies at periodic frequencies to verify that the cleansing technique in place is effective. A correctly chosen worst-case product is key for a science-based technical cleansing program. Most poisonous residue (or residue with lowest pharmacologic dose) is one other consideration. Toxicity (or dosage) is taken into account within the well-known Fourman and Mullen residue calculation. Another consideration in determining the most-difficult-to-cleaning residue in a cleansing matrix is residue "cleanability," or the flexibility of the residue to be cleaned. However, if another related plate (Plate B) from dinner was allowed to rest untouched within the kitchen for several days allowing the residue to dry and harden, the residue would be much more tough to clean. Relating this instance to pharmaceutical cleaning, the cleaning process for Plate B could be much more rigorous than can be required for Plate A to accomplish equivalent cleaning. This analogy has also been used to caution cleansing validation practitioners about rinse samples. A rinse pattern from gear immediately washed after manufacturing is much more meaningful than a rinse sample eliminated after residue had dried for several hours. Many cleansing practitioners evaluate worst-case residues by considering only the solubility and toxicity of the compound of curiosity. The cleanability of products containing polymers, corresponding to controlledrelease tablet products, could also be significantly affected by inactive excipients. For example, contemplate two formulations containing the identical energetic ingredient however having totally different inactive formulations. One may have primarily soluble elements and be quickly dissolving, whereas the opposite is designed to slowly dissolve and provide a chronic launch of drug over an extended time period. The "cleanability" of these two formulations could be markedly totally different even though they include the identical energetic ingredient- with the same solubility and toxicity. However, the knowledge of pharmacological properties of energetic substances can provide extra meaningful data and must be thought-about when establishing a matrix. The output of a cleaning matrix is usually a numerical worth for each lively substance. A threshold value is established, and if this value is exceeded, cleansing validation is required. Another example is as follows: a set-granulation tablet formulation residue is well cleaned when cleansing is initiated immediately after manufacturing is accomplished. However, if residue is allowed to rest in tools over a weekend and dries and hardens, cleaning turns into far more difficult. Note the similarity between this example and the dried spaghetti sauce residue instance described above. Consultation concerning residue "cleanability" with manufacturing personnel who carry out actual cleaning is recommended. Manufacturing personnel are sometimes missed when determining difficult-to-clean dosage varieties. Their input into determining most-difficult-to-clean residues and product groupings may be very useful. Input "from the ground" expressed by individuals who have expertise with doing the actual cleansing coupled with laboratory work, including solubility info, is critical for the dedication of worst-case residues. The mostdifficult-to-clean residue is a key willpower for a cleansing validation program. If worst-case areas on tools pass cleansing validation, it could be assumed that each one other areas on equipment are clear. Identifying worst-case locations on equipment by cautious and deliberate evaluation is important for a cleaning validation program. There is common settlement that most-difficult-to-clean areas of equipment should obtain maximum consideration within the cleaning course of and be appropriately examined (swab or rinse sampling) in cleaning validation. How these locations are determined has not been totally mentioned in the literature. Often alternatives of sampling websites are arbitrary and primarily based on the expertise of a number of people. The strategy to determine the most-difficult-to-clean websites ought to be documented in policy or procedure. Thereafter, the actual determination of sampling websites for every system together with appropriate justification should be documented.
Syndromes
- Sclera
- Receive pain medicine into your veins or into the space that surrounds your spinal cord (epidural)
- Widening of the milk ducts
- Bacterial skin infections
- Bone pain or tenderness
- Make it easier for fluids to pass through small blood vessels
- Head MRI scan
- Persistent cough
- apricots
- Diabetes
Provigil 100 mg buy lowest price
Most crucial or most troublesome cleaning ought to obtain essentially the most testing and highest degree or most frequent evaluation sleep aid lavender oil 200 mg provigil buy overnight delivery. The impression of product and course of adjustments over time could be simply evaluated utilizing graphical representations insomnia 20 generic provigil 200 mg free shipping. Cleaning of extremely potent drugs requiring very low residue ranges for acceptable cleaning could necessitate continued testing until a high stage of confidence within the cleansing process is achieved. For instance, if a selected product required a quantity of re-cleanings through the review period, the cleansing technique in all probability needs to be improved. For example, if modifications to the cleansing process have been wanted as a result of the process was poorly written, the written process should be revised for higher clarity. If equipment appeared to have product residue after cleansing 902 and re-cleaning was beneficial, the extra cleansing should be documented in a deviation. Parenteral Medications Periodic Management Review All data and occasion assessments as described above, together with change control, must be compiled and periodically reviewed by management. The frequency and extent of periodic reviews should be primarily based on product/process threat. Highest threat and most tough cleaning should be more regularly reviewed until a dependable cleaning process history is demonstrated. Thereafter, periodic critiques can be carried out at an appropriate lowered frequency. The administration evaluation process must be documented, and record of management review have to be out there for audit. For instance, certain tools may be more difficult to clean, sure locations on equipment could additionally be harder to clear, or risk evaluation might indicate a high-risk location for cleansing. Cleaning residue information must be rigorously evaluated and never merely judged as move or fail versus acceptance standards. High variation, trends on equipment or on particular sampling areas on tools, or other indicators might suggest the necessity for continued monitoring with analytical testing. While all tools strategies might utilize the same cleansing agent and cleansing process sequence, some equipment could additionally be more difficult to clean than others. For example, an older piece of equipment with a less smooth surface within the course of train might lead to higher residue ranges relative to newer tools. Additional testing on several post-validation lots should confirm the acceptability of the cleaning course of or lack thereof. Change Management and Control A comprehensive change control system is extremely essential in sustaining the validated state of the cleaning course of. Formulation, course of, and gear adjustments at high ranges are often properly communicated and should be appropriately evaluated. Less seen adjustments, nevertheless, such as changes on the operator or mechanic level, could also be missed or thought to be inconsequential. The group ought to develop systems to assure communication of all changes. Evaluation of impact and danger can then be done by responsible and competent personnel. Cleaning performance must be monitored and reviewed after modifications have been applied. A pervasive change management system might eliminate scheduled revalidation of certain processes, once more primarily based on threat analysis. Acceptance Criteria Revision Residue acceptance criteria for equipment surfaces could additionally be calculated by a quantity of approaches. Depending on the pharmacology/toxicity of the drug residue, cleansing floor acceptance standards might or is in all probability not easily achieved. When acceptable ranges of drug residue are simply completed, a lower inner acceptance limit ought to be established to guarantee reliable cleaning course of performance. Stage three Continued Process Verification supplies a periodic review of cleansing performance, nonconformances, deviations, new validation, cleaning validation adjustments, other events, and other data relative to product high quality information typically reviewed within the product Annual Product Review. This evaluate is meant to monitor the respective cleansing validation performance related to product information to decide potential issues with cleansing validation. For example, if evaluation of cleaning a particular product showed repeated nonconformances for dirty tools adopted by deviations to re-clean tools, improvement work to improve the cleansing methodology would likely be indicated. Examples of issues to be addressed throughout Stage 3 evaluate include the following: � Nonconformance: Does a product have an unusual variety of nonconformances indicating equipment was not visually clear after washing Annual Product Review Data Annual product evaluation knowledge and data should also be reviewed for relevance to cleansing. New formulations, formulation adjustments, course of changes, and gear adjustments could all have an impact on cleaning. Such adjustments might have been judged to be insignificant at the time of implementation, however now may be noticed to require corrective action. All adjustments related to cleansing should be reviewed and evaluated by technical personnel, and evaluation should be documented. Less seen changes, such as changes at the operator or mechanic degree, could additionally be ignored or thought to be inconsequential. For example, mechanics might substitute plastic part items with out regard to compatibility with the cleaning agent. Corporate Policy Documents Large multisite organizations have a corporate-level coverage document that typically describes the cleansing validation program on the websites. These are basic documents that describe the company approach to cleaning validation. They should generally describe a complete lifecycle strategy, including design and improvement, demonstration in conformance heaps, and postvalidation monitoring and maintenance. Other particular company necessities, policies and procedures, company templates, and regulatory requirements and expectations may be included in documentation at this level. Other site documents such as the product cleansing matrix, equivalent tools, surface space calculations, and cleaning gear qualification are listed with references for retrieval. These high-level documents provide the policy and apply bases beneath which actual cleansing validation is performed. All work related to cleansing validation within the respective levels of the validation lifecycle must be documented. The following related to cleaning and cleaning validation documentation are addressed: � Scope of cleansing validation documentation: the lifecycle method to course of validation has expanded the scope and expectations for cleansing validation documentation. Cleaning validation documentation consists of high-level coverage documents, cleansing course of development stories, cleaning validation conformance heaps, post-validation monitoring, and upkeep documents, together with change control, and other related paperwork. Scope of Cleaning Validation Documentation Cleaning validation documentation consists of high-level coverage and practice paperwork, specific cleansing validation lifecycle 904 Parenteral Medications concerned. Working-level paperwork corresponding to particular cleansing validation plans, protocols, and results demonstrate the scientific and technical approach in the cleansing validation design and execution.
Proven 200 mg provigil
The appearance of major degradation merchandise additionally commonly follows pseudo-zero-order kinetics sleep aid no side effects provigil 200 mg generic on-line, except the products themselves degrade sleep aid video provigil 100 mg discount mastercard, during which case more complex fashions may need to be thought-about [76]. Data for submission are expected to be correctly tabulated and reviewed, with outcomes and developments for every attribute individually mentioned, as appropriate. The shelf life for the product should not exceed the shelf life predicted for any individual attribute. The mixture of accelerated, intermediate, and long-term stability knowledge are first evaluated for significant change. Little Change over Time or Little Variability Yes-both accelerated and long term No-accelerated or long run Statistical Analysis Not required Yes No Yes-Accel. Parenteral Product Specifications and Stability more important fractions of the initial content, so alternate kinetic fashions may also be required in these circumstances. For simplicity, the overview of statistical therapy offered here will focus on zero-order kinetic models, but most of the concepts may be extended to alternate kinetic models through variable transformations or nonlinear regression modeling. Linear regression as utilized for analysis of zero-order trends in stability information for a single batch is a simple utility of linear regression as described in introductory texts on applied statistics [77,78]. To briefly define the method, think about a series of assay values Yx(ti) for batch x which are collected over a set of time intervals, ti similar to stability time points I = 1. The "true" shelf life for the batch has equal chance of being longer or shorter than 30 months as a outcome of the variability in the knowledge limits the diploma of certainty in estimate of the shelf life. The greater the variation of knowledge concerning the line, the greater the diploma of uncertainty. Clearly, since merchandise are anticipated to meet specs all through shelf life, the regression line estimate of shelf life is most likely not your greatest option as a end result of it is extremely likely (50% probability) to overestimate the true shelf life. Assuming the errors are usually distributed, confidence limits could be calculated primarily based on standard formulae. These limits estimate the region inside which the true pattern line for the batch is anticipated to lie at a selected level of confidence. This distinction is the penalty of uncertainty related to each the limited quantity of data and the extent of future extrapolation. Although they comply with a common zero-order trend, deviations of individual factors concerning the line are frequent. The errors, are assumed to be normally distributed, with imply of zero and commonplace deviation of. Assuming Model 1 is predictive, the equation can be utilized to calculate the expected assay end result at occasions apart from these the place the assay was tested, including future times. As an instance, consider the steadiness information for an assay take a look at collected for certainly one of three main stability batches (Batch A). The dashed line is the lower 95% two-sided confidence interval for the regression line. Where batch data pooling could be justified, primarily based on a set of applicable choice standards, more extended shelf lives can regularly be justified, and in most cases, more practical shelf life estimates could be obtained. As a typical example of how this procedure is applied to batch pooling, examine Model 1, the place slope and intercept are allowed to differ by batch, to Model 2, the place the intercept varies by batch, but all batches share a standard slope. On the other hand, if variations in the slopes over the size of the road phase seem small relative to the distribution of factors in regards to the line, it would appear unlikely that the differences would contribute a lot to the overall match of the mannequin. In this case, the slopes could be pooled without loss of priceless info relating to batch-to-batch differences in stability habits. When the info are distributed normally about the regression lines, this intuitive evaluation could be readily translated into a take a look at statistic constructed from the mean squared errors for every model, and values of the statistic could be in contrast with proportion factors of standard F-distribution to assess the statistical significance of model enhancements as further independent slope phrases are added. Similar approaches can be utilized to compare stability tendencies across formulation, course of, or packaging changes. The confidence interval has additionally narrowed, partially because of the larger number of data points included within the fitted model. For this example, pooling when allowed, will increase the estimated shelf life from 22 to 27 months. The dashed line is the lower 95% two-sided confidence interval for the regression line of Batch A. The dotted line is the lower 95% two-sided prediction interval for the regression line of Batch A. Parenteral Product Specifications and Stability Two caveats should to be saved in thoughts. Possible variations in future batches need to be considered when release criteria are established, and the relationship between expiration period, shelf-life specs, and launch specifications have to be understood. The 95% confidence interval used to estimate the shelf life reflects confidence within the estimation of linear model parameters, and this confidence interval shrinks because the number of factors included in the analysis will increase. Matrix designs commonly embrace combinations of factors such as power, container dimension, container type, and even minor modifications in formulation. Where a full factorial design would require testing of all mixtures at all time points, a matrixed design allows for a subset of the samples to be tested at many of the time factors. Depending on the product and testing schedule, stability testing may be lowered by as a lot as 30%�50% or extra, a considerable decrease for designs that include multiple factors. Briefly, the most important points are as follows: � Data from three main batches must be positioned on formal stability research of at least 6 months duration on the selected long-term storage situation. Accelerated stability studies are beneficial to help characterize the degradation profile and help excursions, however accelerated circumstances might not mirror actual product stability or degradation traits at storage circumstances. These practices enable for some total reduction in the stability testing workload. Bracketing is the practice of testing stability for products on the extremes of strength or container dimension and using the results to support the expiration period for the product at intermediate energy or container measurement. If the excessive and low strengths differ in stability, the expiration period of the intermediate energy is constrained to the shorter of the 2 expiration durations. Some care is clearly required for multiphase merchandise like suspensions, where changes in energy may be related to change within the distribution of drug among phases that differ in stability. Specifications: take a look at procedures and acceptance standards for new drug substances and new drug products: chemical substances, step 4 model. Specifications: check procedures and acceptance criteria for biotechnological/biological products, step four version. Guidance for business: allowable excess quantity and labeled vial fill measurement in injectable drug and organic merchandise. Parenteral Quality Control: Sterility, Particulate, and Package Integrity Testing. Guidance for trade: submission of documentation in purposes for parametric launch of human and veterinary drug merchandise terminally sterilized by moist warmth processes. Guidance for trade: container and closure system integrity testing in lieu of sterility testing as a part of the steadiness protocol for sterile merchandise. Container closure integrity testing � practical aspects and approaches in the pharmaceutical business.

Discount provigil 200 mg without prescription
Health careassociated an infection after red blood cell transfusion: a scientific evaluate and meta-analysis sleep aid 88 cents purchase provigil 100 mg with amex. Duration of pink blood cell storage is related to increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic accidents sleep aid neuropathy treatment group sleep aid provigil 200 mg order with visa. Efficacy and security of dopamine versus norepinephrine in the administration of septic shock. Effect of sodium bicarbonate administration on mortality in patients with lactic acidosis: a retrospective evaluation. Describe the pathophysiology and scientific presentation of acute and persistent bronchial asthma. Despite variability in the severity of continual asthma, all sufferers with bronchial asthma are susceptible to acute extreme disease. International guidelines emphasize the importance of treating underlying airway inflammation to management asthma and reducing asthma-associated dangers. There seems to be an inherited element as a result of the presence of asthma in a mother or father is a strong threat issue for creating asthma in a child. The presence of atopy is a powerful prognostic issue for continued asthma as an adult. Although asthma happens early in life for most patients, those with occupational bronchial asthma develop the illness later upon publicity to particular allergens within the workplace. A key function of the pathophysiology is airway hyperresponsiveness, which is exaggerated narrowing of the airways in response to a trigger or allergen corresponding to chilly air, sturdy odors, pollen, or mud. Airway narrowing outcomes from contraction of airway smooth muscle, increased mucus secretion, airway edema, and reworking. This results in B-cell production of antigen-specific immunoglobulin E (IgE), pro-inflammatory cytokines (eg, interleukin-5), and chemokines that recruit and activate eosinophils, neutrophils, and alveolar macrophages. In the late-phase response, activated airway cells launch inflammatory cytokines and chemokines, thereby recruiting more inflammatory cells into the lungs. The late-phase response happens 4 to 6 hours after the preliminary allergen problem and results in less intense bronchoconstriction in addition to elevated airway hyperresponsiveness and airway inflammation. Diagnosis is predicated on an in depth medical history, physical examination of the upper respiratory tract and skin, and spirometry. Risk refers to the potential for future extreme exacerbations and asthma-related demise, progressive loss of lung function (adults) or reduced lung development (children), and the incidence of drug-related antagonistic results. Acute Asthma In acute bronchial asthma, symptoms of wheezing, coughing, and shortness of breath improve. Chronic Asthma In continual bronchial asthma, initial classification of asthma severity is based on present illness impairment and future risk. Symptoms � Patients normally complain of wheezing, shortness of breath, coughing (usually worse at night), and chest tightness. In acute severe asthma, sufferers may be unable to communicate in full sentences. Laboratory Tests � Spirometry may reveal airway limitation and excessive airway variability. Other Diagnostic Tests � Full pulmonary operate exams must be carried out at baseline in sufferers 5 years or older to rule out different issues. Nonpharmacologic Therapy Incorporating nonpharmacologic care and education is essential for patients with asthma. Components of schooling involve bronchial asthma trigger avoidance, correct administration of inhaled medications, and asthma selfmanagement (Table 14�1). He has had oral prednisone bursts twice prior to now 3 months with aid of his symptoms. He has tried using ranitidine and omeprazole in the past with no improvement in his nighttime cough. He tried using nasal corticosteroids for his allergy symptoms and sinuses with no aid. Pharmacologic Therapy Treatment of persistent bronchial asthma involves avoidance of triggers known to precipitate or worsen asthma and use of long-term management and quick-relief medicines. Adverse effects of 2-agonists embrace tachycardia, tremor, and hypokalemia, that are normally not troublesome with inhaled dosage forms. Because of the lengthy Drug Delivery Devices Direct airway administration of asthma medicines through inhalation is probably the most environment friendly route and minimizes systemic adverse results. Selection of the appropriate inhalation system depends on affected person characteristics and drugs availability (Table 14�2). Poor inhaler technique ends in elevated oropharyngeal deposition of the drug, resulting in decreased efficacy and increased antagonistic effects. Shake the inhaler nicely before use Remove the cap Exhale away from the inhaler Tilt your head again slightly and place the mouthpiece into the mouth with lips sealed tightly around the inhaler 5. Press down on the inhaler to launch medication as you begin to breathe in slowly. Hold your breath for as long as attainable, up to 10 seconds to allow the drugs to reach deeply into your lungs 7. Bring the spacer to your mouth, put the mouthpiece between your tooth, and close your lips round it 6. Hold your breath for about 10 seconds, then breath out Use a spacer with mask for children under 5 years old, or for these unable to maintain their breath. Place mouth on the mouthpiece and breathe out and in slow and steadily into the mouthpiece 1. Formoterol and vilanterol are available only together with inhaled corticosteroids (Table 14�4). Combination products might enhance adherence because of the necessity for fewer inhalers and inhalations. Corticosteroids Corticosteroids are probably the most potent anti-inflammatory agents obtainable for asthma remedy and can be found in inhaled, oral, and injectable dosage varieties. They lower airway inflammation, attenuate airway hyperresponsiveness, and reduce mucus production and secretion. Maximum of fluticasone/salmeterol 100/50 mcg, 1 inhalation twice every day is permitted in kids ages 4�11 years with out specification of low, medium, or high dosage. Product choice is based on preference for dosage type, delivery device, and cost. Longer treatment could also be essential to notice the full effects on airway inflammation. For most delivery units, the majority of the drug is deposited in the mouth and throat and swallowed. For this reason, systemic corticosteroids are started early in the midst of acute exacerbations.
Buy cheap provigil 100 mg online
At a given incident energy density insomnia novel provigil 100 mg low price, a high mass absorption coefficient will result in insomnia buy generic provigil 200 mg line a higher dose fee than a low mass absorption coefficient. The equation for dose price is given as the product of the previous two variables. At equal output powers, gamma irradiators have the lowest dose charges, X-ray irradiators greater dose rates, and electron beam irradiators the very best dose rates. By means of comparability, if the dose fee in a gamma irradiator had been normalized to 1, dose fee in an X-ray irradiator can be approximately 10 or extra, and dose rate in an electron beam irradiator would be higher than 100. For a given modality of irradiation, varied strategies can be found for controlling the dose charges that are delivered to a product. For example, reducing the output energy of the Radiation Sterilization irradiator offers one method for decreasing the dose fee. In the case of a gamma irradiator, this may entail loading less isotope within the source plane(s), and for electron beam and X-ray irradiators, it could probably be completed by merely dialing down the present of the accelerator. Placement of a shield between the supply and the goal is one other technique for reducing the dose price. In a gamma irradiator, one can take benefit of the isotropic nature of the radiation area and easily move the product further from the supply, which is in a position to cut back the facility density incident on the target. If dose price is considered an important parameter within the irradiation of a selected pharmaceutical/medical product, choice of the modality of radiation that best meets the dose rate necessities ought to be taken into consideration at an early level within the sterilization project. To satisfy technical criteria for irradiation of the product, a minimum of the minimum dose have to be delivered to the product unit. For high-density merchandise and those merchandise that are extremely heterogeneous in nature, photon radiation- whether or not gamma or X-ray-may be preferred to high-energy electrons. The penetration of high-energy photons in supplies is described by the product of an exponential issue and a semiempirical buildup issue that accounts for scattering of the photons. Product may be transported across the source airplane within the outer two passes solely, which considerably increases the standoff distance of the totes from the source airplane, thus reducing the impact of geometric attenuation on dose distribution. In irradiators which might be designed for precision dosing of product, corresponding to those utilized in dose validation research, the irradiation containers are sometimes offset farther from the source than the standoff distance found in production irradiators. As seen from this figure, at the completion of the process cycle, both sides of the tote have been uncovered to equal quantities of radiation. In homogeneous materials, the depth-dose profile of highenergy electrons in materials is a well-characterized parameter. The proven truth that the absorbed dose is greater contained in the target than on the floor where the electrons are incident is due to scattering of the electrons as they penetrate deeper into the goal. The precipitous falloff in absorbed dose at deeper penetrations into the goal happens after the electrons have given up most of their power in inelastic scattering collisions. Of the three modalities for irradiation, an X-ray irradiator offers the potential of delivering probably the most uniform dosing to product. Depending on the utmost vitality of the X-rays, the mass absorption coefficient could be lower than that of cobalt-60 photons. For this cause, shielding is less of a priority in X-ray irradiators than in gamma irradiators. However, geometrical effects will come into play at boundaries of the product unit due to the beam properties of the X-ray radiation. In this way, photons also can scatter into the fabric as nicely as out of the material. Parenteral Medications Temperature the rise in temperature of irradiated merchandise relies on three basic parameters. Because the power absorbed per unit mass of fabric is the same as absorbed dose, it follows that higher absorbed doses ought to lead to higher excursions in temperature. At high dose charges, the material might not have sufficient time to thermally chill out, thus leading to larger temperatures in the irradiated product. The final parameter that can considerably affect product temperature is related to the thermal properties of the irradiated material. For equal irradiation situations, materials that are good conductors of warmth with related specific heats ought to expertise a smaller enhance in temperature than supplies with excessive thermal resistance. Let us think about every of those parameters and its potential impact on product temperature. A decrease absorbed dose equates to a lower quantity of vitality absorbed per unit mass and a smaller improve in temperature. In adiabatic heating, the change in temperature is given by the next relationship in Equation 34. If the ambient temperature is roughly 30�C, the product temperature on this instance may attain 54�C. Therefore, given the best situations, vital will increase in temperature can happen in irradiated products. For some products such as proteins the place temperature might have a significant impression on the process, an increase in temperature of this magnitude may have an important impact on the response of the protein. The risk of adiabatic heating is bigger in excessive dose fee environments. There are numerous ways to management the temperature and mitigate its impact on the irradiated product. The second technique of control is to irradiate the product in a low dose price surroundings. An further consideration would involve refrigeration of the product so the preliminary temperature is beneath the ambient value. Because the thermal diffusivities of typical pharmaceutical products and medical devices are relatively low. Although little could be accomplished to remedy this condition, it could be attainable to improve heat flow by acceptable selection of packaging materials and other supplies that may encompass the product unit. In this regard, elimination of packing materials corresponding to Styrofoam which will encase the product or substitute with a extra environment friendly heat-conducting materials could additionally be beneficial. Establishing the Sterilization Dose and Maximum Dose Inactivation of Microorganisms the biocidal effect on microorganisms from publicity to radiation is nicely documented [10,11]. The variety of surviving microorganisms decreases with a rise within the absorbed dose. The dose�survivor curve, which plots survivors versus dose, can take on totally different shapes, however a common shape obeys first-order kinetics and follows an exponential decrease in surviving microorganisms with dose [12]. This parameter, which could be extracted from the warmth conduction equation, 744 reduce the variety of survivors by 1 log or an element of 10. This parameter is referred to because the D10 worth, and for microorganisms that sometimes reside on pharmaceutical merchandise and medical units, D10 values vary from lower than 1 kGy up to several kGy. Both parameters depend on the manufacturing circumstances, which outline the type and stage of microbial contaminants on the manufactured unit. Parenteral Medications extra proof against radiation than the pure bioburden that might be current on the product. In Method 2, information is obtained in regards to the resistance to radiation of the pure bioburden present on the product. A total of 10 product items are chosen from every of three independent product batches and these 30 product objects are tested for bioburden. In these instances the place manufacturing is limited to a single batch, solely 10 product objects need to be tested for bioburden.
Order provigil 200 mg without a prescription
In spontaneously breathing sufferers sleep aid 25mg doxylamine succinate provigil 200 mg generic otc, respiratory alkalosis is usually solely gentle or average in severity and no particular remedy is indicated insomnia wheesung 200 mg provigil cheap mastercard. Severe alkalosis usually represents respiratory alkalosis imposed on metabolic alkalosis and may enhance with sedation or rebreathing maneuvers (rebreathing mask, paper bag). Patients receiving mechanical air flow are treated with reduced minute ventilation achieved by reducing the respiratory fee and/or tidal volume. If the alkalosis persists within the ventilated affected person, high-level sedation or paralysis is efficient. Collect Information: � Every affected person with a suspected acid�base disturbance ought to have an arterial blood fuel and a serum chemistry panel drawn concurrently. The results of these checks ought to be reviewed using a scientific approach to guarantee correct interpretation. Develop a Care Plan and Implement the Care Plan: � Continuous cardiovascular and hemodynamic monitoring must be used for significant pH disturbances because the most serious sequelae of acid� base issues include electrolyte abnormalities, cardiac dysrhythmias, and hypotension. Definitive remedy for these disturbances requires treatment of the sickness that has disrupted the pH equilibrium. Although supportive therapy is commonly required for profound acid� base disturbances, definitive therapy must target the underlying process that has led to the noticed derangements. This should result in an evaluation of which deviations symbolize the primary abnormality and which symbolize compensatory changes. The anion gap and the surplus hole are helpful instruments that may determine hidden issues. The function of the anion hole in detecting and managing blended metabolic acid�base disorders. The use of the urinary anion hole within the analysis of hyperchloremic metabolic acidosis. This sort is often familial and follows an autosomal dominant sample in roughly 50% of instances. Advocacy organizations, such as the Alzheimer Association, can present early schooling and social assist for each the patient and household. The Alzheimer Association has developed a listing of common warning indicators, which embody memory loss, problem finishing acquainted tasks, disorientation, issues with word finding, misplacing issues, impaired judgment, social withdrawal, and changes in mood. Of these affected, one in ten are sixty five years of age or older, and one in three are 85 years of age or older. Cholesterol increases -amyloid protein synthesis, which might lead to plaque formation. In early phases of the disease, neurodegeneration of areas answerable for cholinergic transmission ends in a cholinergic deficit. As the illness progresses, widespread neurodegeneration causes imbalance in all neurotransmitter methods. These are primarily positioned in mind regions concerned in studying, reminiscence, and emotional behaviors such as the hippocampus within the medial temporal lobe of the cerebral cortex, basal forebrain, and amygdala. Although criteria are still being evaluated to understand the levels of cerebrovascular pathologies that contribute to cognitive impairment, epidemiological studies have discovered that the presence of vascular and metabolic danger components during midlife are most strongly related to threat of cognitive impairment and dementia. The neurons that provide a lot of the cholinergic innervation to the cortex are prominently affected. Currently, there are biological Plaques the A protein is current in neuritic plaques in a unhazardous, soluble kind in human brains. Inflammation occurs secondary to clusters of astrocytes and microglia surrounding these plaques. Blocking acetylcholinesterase, the enzyme that degrades Ach within the synaptic cleft, results in an elevated degree of Ach with a aim of stabilizing neurotransmission. It affects cognition by way of facilitation of connections with cholinergic neurons in the cerebral cortex and basal forebrain. Evidence of significant cognitive decline from a earlier degree of performance in one or more cognitive domains (complex consideration, govt perform, learning and reminiscence, language, perceptual-motor, or social cognition) based on: 1. Concern of the person, a educated informant, or the clinician that there has been a major decline in cognitive function and a couple of. A substantial impairment in cognitive efficiency, ideally documented by standardized neuropsychological testing or, in its absence, one other quantified scientific assessment B. Insidious onset and gradual progression of impairment in one or more cognitive domains (for main neurocognitive disorder, at least two domains have to be impaired) C. Criteria are met for either probable or potential Alzheimer disease as follows: For Major Neurocognitive Disorder: Probable Alzheimer disease is identified if either of the following is current; otherwise possible Alzheimer disease must be identified. Evidence of a causative Alzheimer disease genetic mutation from household history or genetic testing 2. Clear proof of decline in memory and learning and a minimal of one other cognitive domain b. Evidence of modest cognitive decline from a earlier degree of efficiency in a number of cognitive domains (complex consideration, government operate, studying and reminiscence, language, perceptual-motor, or social cognition) based on: 1. Concern of the individual, a knowledgeable informant, or the clinician that there has been a light decline in cognitive operate and a pair of. A modest impairment in cognitive efficiency, preferably documented by standardized neuropsychological testing, or in its absence, another quantified scientific assessment B. Impairment in motor, behavioral, and sensory functioning occurs later in the illness. Assess all comorbid medical problems and drug therapies that will have an effect on cognition three. Secondary goals include treating psychiatric and behavioral signs which will occur in the course of the course of the illness. Additionally, psychiatric and behavioral symptoms that happen in the course of the course of the disease must be handled as they happen. Treatment choices, legal and financial decisions, and course of the sickness should be mentioned with the affected person and family members. The number of one ChE over another must be based on variations in mechanisms of action, antagonistic reactions, titration schedules, potential drug interactions, and patient and caregiver preference. Patients ought to be switched to another ChE inhibitor from their preliminary ChE inhibitor if they show an initial lack of efficacy, initially reply however then lose medical profit, or expertise safety/ tolerability issues. The change also needs to be primarily based on practical expectations of the affected person and/or caregiver. The 23-mg dose confirmed a small enchancment in cognitive symptoms compared to the 10 mg/day dose; nevertheless, there was no enchancment in general functioning, and there was a better incidence of adverse effects. Namzaric, a fixed-dose combination of extended-release memantine and donepezil (14/10 mg and 28/10 mg), was just lately approved. If unwanted side effects cause intolerance, a quantity of doses may be held, after which dosing can be restarted on the similar or subsequent lower dose. Galantamine can also improve glutamate and serotonin ranges, however whether this brings additional benefit is unknown. Similar to donepezil, if inhibitors are given concurrently with galantamine, increased cholinergic side effects must be monitored. Closer monitoring ought to be carried out if memantine is given concurrently with a ChE inhibitor. Mechanisms by which these compounds are thought to work include7: � Reducing levels of mind A or manipulating its configuration � Targeting proteins Patient Encounter Part 2 the affected person returns to the clinic in 6 months.